Arrange a Dermal Filler Consultation with Dr. Laura Geige Today
Risks Associated with Temple Fillers
Risks associated with temple fillers are a significant concern for many individuals considering this cosmetic procedure. One of the most notable risks is blindness, which can occur due to the close proximity of the temple filler injection site to the ophthalmic nerve. This nerve runs close to the surface of the eye and can be easily damaged during the injection process, leading to vision loss or blindness.
The ophthalmic artery also passes near the injection site, increasing the risk of blood vessel damage or vascular occlusion, which can cause permanent vision loss. In some cases, the temple filler may dissolve and absorb into the bloodstream, potentially leading to anaphylaxis or other systemic reactions.
Other complications that have been reported with temple fillers include diplopia (double vision), eye pain, and ptosis (drooping eyelids). In rare cases, the filler material may migrate to other parts of the face or body, causing a range of symptoms including swelling, redness, and scarring.
The causes of blindness associated with temple fillers can be attributed to several factors. One major cause is incorrect injection technique, which can lead to nerve damage or vessel occlusion. Another factor is the use of poorly designed or non-sterile filler products, which can increase the risk of infection or other adverse reactions.
Elevated pressure within the orbital cavity, caused by the presence of a temple filler, can also contribute to eye damage. This increased pressure can cause the eye to become stretched or compressed, leading to permanent vision loss. In some cases, the pressure may be so severe that it causes the **eyelid** or other facial tissues to become damaged.
Another risk associated with temple fillers is nervous system damage, particularly when performing the procedure without proper training or experience. This can lead to numbness, tingling, or other sensory disturbances in the face, as well as more severe complications such as palsy (paralysis) of the facial muscles.
The risk of blindness associated with temple fillers is often linked to overfilling or underfilling, which can both lead to increased pressure within the orbital cavity. Overfilling can cause a buildup of pressure, leading to eye damage and vision loss, while underfilling may result in inadequate support for the eyelid or other facial tissues.
To minimize the risks associated with temple fillers, it is essential to choose a qualified and experienced healthcare professional who has performed numerous procedures without incident. A thorough pre-procedure consultation and post-operative care plan can also help to identify and mitigate potential complications.
Additionally, individuals should carefully weigh the benefits of the procedure against the potential risks and complications. A comprehensive understanding of the potential outcomes and any associated risk factors is essential for making an informed decision about undergoing temple filler treatment.
Risk of blindness associated with temple fillers, specifically granuloma formation, is a significant concern that needs to be addressed.
Temple fillers, also known as facial fillers, are non-surgical cosmetic procedures used to restore lost volume and enhance facial features.
Granulomas are abnormal growths of tissue that can form in response to foreign substances, including fillers.
In the context of temple filler injections, granuloma formation is a rare but potentially serious complication.
The risk of granuloma formation with temple fillers is thought to be related to the type and brand of filler used, as well as individual patient factors.
Common types of facial fillers linked to granuloma formation include those made from hyaluronic acid (e.g., Restylane, Juvederm), calcium hydroxylapatite (Radiesse), and poly-L-lactic acid (Sculptra).
The symptoms of a granuloma can range from mild discomfort and redness to severe pain, swelling, and vision loss.
One of the most alarming manifestations of granuloma formation is its potential to cause visual disturbances, including blurred vision, double vision, or even blindness.
Blindness associated with temple fillers has been reported in a small number of cases, often as a result of advanced granulomatous inflammation that extends beyond the site of the filler injection.
The exact incidence of blindness caused by temple fillers is difficult to determine due to variations in reporting and diagnostic criteria.
However, studies suggest that the risk of serious complications from facial fillers, including granulomas leading to visual loss, is relatively low when proper technique and precautions are employed.
Causes of blindness associated with temple fillers can be attributed to several factors, including:
1. Inadequate injection technique or anatomy-related challenges.
2. Use of high-dose filler materials.
3. Presence of underlying medical conditions (e.g., rosacea, eczema).
4. Poor post-treatment follow-up and monitoring.
Prevention is key in minimizing the risk of complications from temple fillers, including granuloma formation and associated vision problems.
Patients should carefully select a qualified healthcare provider with extensive experience in facial filler procedures and follow all post-treatment instructions to ensure optimal outcomes.
Regular follow-up appointments with their practitioner will help identify any potential issues promptly, reducing the risk of complications like granuloma formation or visual disturbances.
Moreover, patients should be aware of the warning signs of adverse reactions after a temple filler procedure, including sudden vision changes, eye pain, redness, swelling, and bruising.
By understanding the potential risks associated with temple fillers and taking proactive steps to minimize these risks, individuals can make more informed decisions about their cosmetic care.
This includes thoroughly researching healthcare providers, carefully selecting filler materials, and adhering to post-treatment guidelines to ensure a safe and successful outcome.
Ultimately, patients should prioritize open communication with their healthcare provider about any concerns or complications they experience after temple filler treatment.
The formation of **granulomas** is a primary concern associated with temple fillers, as they can cause permanent vision loss. These clusters of inflammatory cells can develop in the eye and lead to significant visual impairment.
According to a study published in the Journal of Ophthalmology, there is a link between temple fillers and the development of granulomas. The researchers found that individuals who underwent temple filler injections were at a higher risk of developing granulomas, which can lead to vision loss.
The risks associated with temple fillers are multifaceted:
- **Granuloma formation**: This is the primary concern, as it can cause permanent vision loss. Granulomas are clusters of inflammatory cells that can develop in the eye and lead to significant visual impairment.
- Visual field defects: The development of granulomas can also lead to visual field defects, which can affect an individual’s ability to see objects or people out of their peripheral vision.
- **Eye inflammation**: Temple fillers can cause eye inflammation, which can lead to a range of complications, including granuloma formation and vision loss.
The potential risks associated with temple fillers are significant, and individuals should carefully weigh the benefits against the potential drawbacks before undergoing treatment. It is also essential to follow proper post-injection care and attend regular follow-up appointments with an eye doctor to monitor for any adverse effects.
Some common symptoms of granuloma formation include:
- Blurred vision
- Double vision
- Fatigue
- Pain or tenderness in the eye
If you are considering temple fillers and are concerned about the risks of granuloma formation, it is essential to discuss your individual situation with an eye doctor. They can help you understand the potential benefits and risks of treatment and determine if temple fillers are right for you.
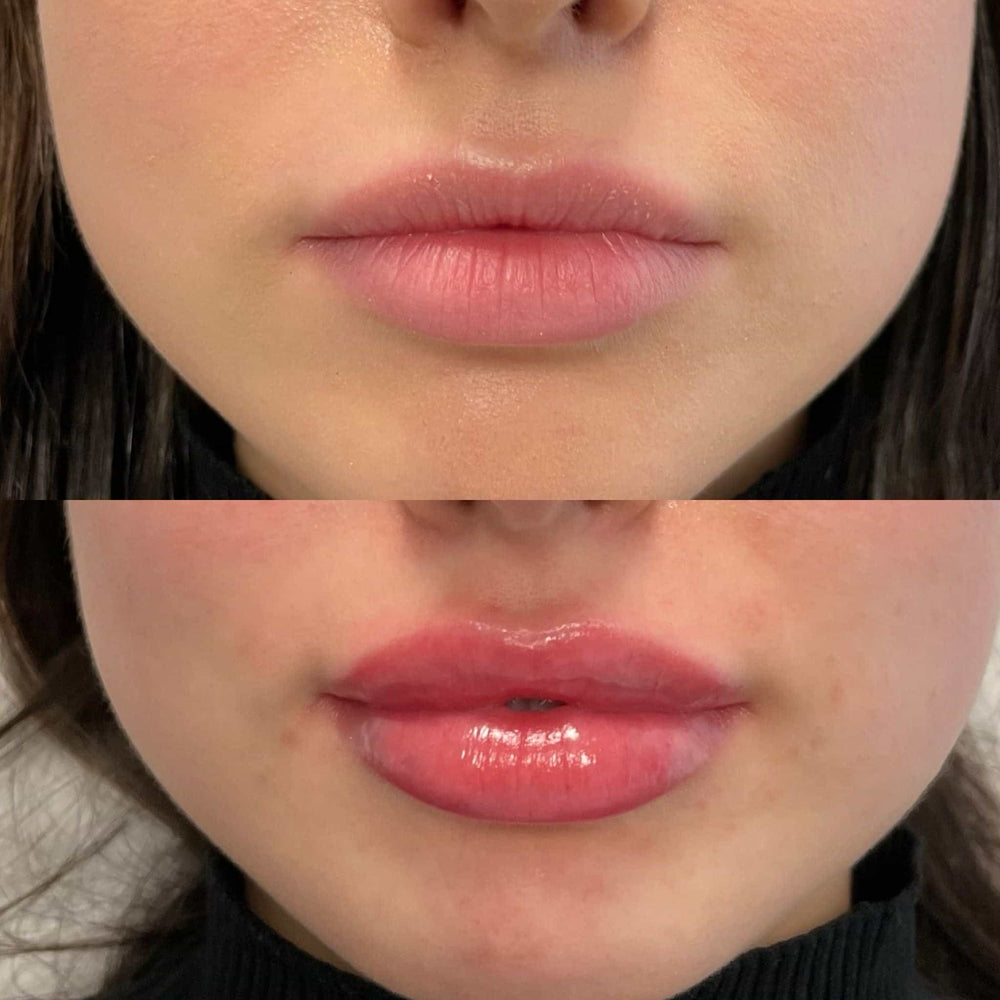
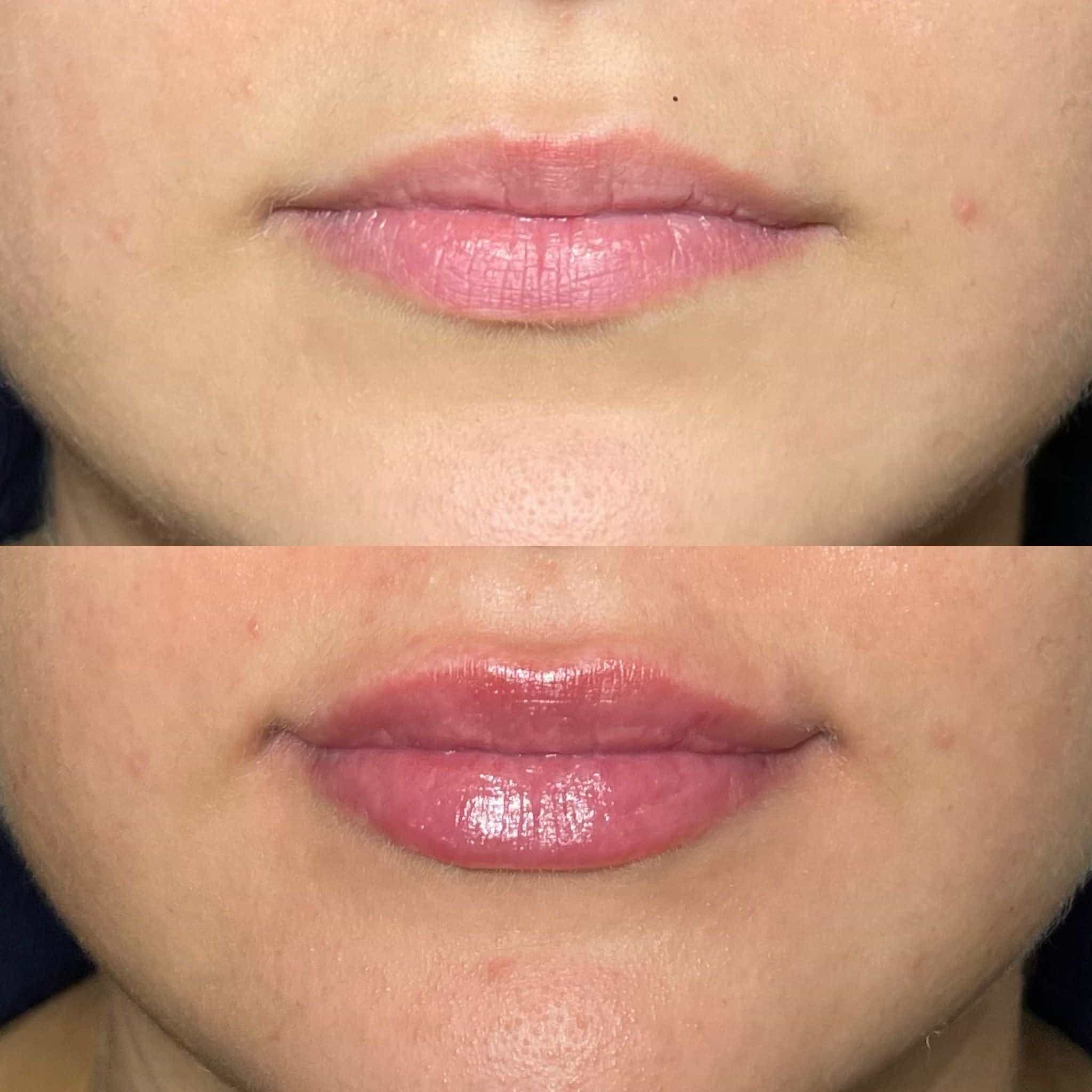
The temple filler, also known as a dermal filler, is a cosmetic treatment used to restore volume and smooth out wrinkles and fine lines around the eyes and temples. However, like any medical treatment, it carries potential risks and complications.
One of the most significant risks associated with temple fillers is damage to the optic nerve. The optic nerve is responsible for transmitting visual information from the eye to the brain, and any injury to this nerve can cause permanent vision loss or blindness.
The risk of optic nerve damage is particularly high when using hyaluronic acid (HA) dermal fillers, which are the most commonly used type of temple filler. HA fillers are injected into the soft tissue around the eyes and temples, and they can cause inflammation and swelling in this area. If the filler material is too thick or if it is not properly dissolved, it can put pressure on the optic nerve.
According to a study published in the Journal of Clinical and Aesthetic Dermatology, the incidence of vision loss or blindness after HA dermal filler injection is estimated to be around 1 in 100,000 to 1 in 200,000 treatments. However, this risk can be significantly increased if the filler material is injected too close to the optic nerve.
Other risks associated with temple fillers include:
Schedule a Dermal Filler Appointment with Dr. Laura Geige at It’s Me and You Clinic
• Infection: As with any invasive medical treatment, there is a risk of infection with temple fillers. This can be caused by bacteria or other microorganisms that are introduced into the skin during the injection process.
• Allergic reactions: Some people may be allergic to the ingredients in temple fillers, which can cause a range of symptoms including hives, itching, and swelling.
• Bleeding or bruising: Temple filler injections can cause bleeding or bruising at the injection site, especially if the skin is thin or fragile.
• Asymmetrical results: If the filler material is not evenly distributed around the eyes and temples, it can cause asymmetrical results that may be noticeable.
• Long-term complications: While rare, long-term complications such as scarring, nodules, or lumps can occur with repeated temple filler injections.
To minimize the risks associated with temple fillers, it is essential to:
• Choose a qualified and experienced healthcare professional who has performed many temple filler treatments.
• Follow all pre- and post-treatment instructions carefully.
• Have realistic expectations about the results of the treatment.
• Be aware of any potential risks or complications associated with the treatment.
It is also essential to discuss your medical history, including any previous eye surgery or vision problems, with your healthcare professional before undergoing temple filler treatments.
The risks associated with **temple fillers** are a topic of great concern, and one of the most severe and potentially permanent complications that can arise from this procedure is damage to the optic nerve.
Optic neuropathy, which is damage to the optic nerve, can cause vision loss and even blindness. According to the American Academy of Ophthalmology, temple fillers can indeed lead to optic neuropathy, resulting in a range of vision problems that can be severe enough to cause permanent blindness.
One of the most alarming aspects of this risk is that it can occur without any noticeable symptoms or warning signs until significant damage has been done. This means that individuals who undergo temple filler procedures may not even realize they are experiencing optic nerve damage until it’s too late, and irreversible vision loss has occurred.
In order to better understand the risks associated with temple fillers and optic neuropathy, let’s take a closer look at some of the other complications that can arise from this procedure:
- Visual Disturbances: In addition to optic neuropathy, temple fillers can also cause visual disturbances such as blurred vision, double vision, and sensitivity to light.
- Eye Pain and Discomfort: Some individuals may experience eye pain or discomfort after undergoing a temple filler procedure, which can range from mild to severe.
- Infection and Abscesses: As with any invasive medical procedure, there is a risk of infection or abscess formation at the site of insertion. If left untreated, these complications can lead to serious health consequences.
- Temporary Blindness: In some cases, temple fillers can cause temporary blindness or vision loss, although this is usually reversible once the filler has dissolved or been removed.
The risks associated with temple fillers are significant, and it’s essential for individuals considering this procedure to carefully weigh the potential benefits against the potential drawbacks. By being aware of the possible complications that can arise from temple fillers, individuals can make informed decisions about their healthcare and take steps to minimize the risk of adverse outcomes.
Prevention and Treatment Options
Prevention is key to avoiding complications from temple filler, a cosmetic treatment used to temporarily fill in facial creases and folds. To minimize the risk of adverse effects, it is essential to choose a qualified and experienced practitioner, who will follow proper sanitation and safety protocols.
The most common complications associated with temple filler include:
- Blindness or vision loss
- Allergic reactions or anaphylaxis
- Pain, redness, swelling, and bruising at the injection site
- Infection or abscess formation
- nasal congestion or sinus problems
- Temporary or permanent nerve damage
- Cerebrospinal fluid leak or hemorrhage
A thorough medical evaluation, including a review of medical history and a physical examination, is crucial before undergoing temple filler treatment. This helps identify any underlying conditions that may increase the risk of complications.
During the treatment process, the practitioner should use sterile equipment and follow proper injection techniques to minimize the risk of adverse effects. The area around the injection site should be cleaned and disinfected before the procedure.
To reduce the risk of blindness or vision loss, it is essential to choose a qualified and experienced practitioner who has received specialized training in administering temple filler injections. The practitioner should also use high-quality products and follow established guidelines for dosing and administration.
The treatment itself typically involves administering a local anesthetic to numb the area, followed by the injection of a temporary filler material, such as hyaluronic acid or calcium hydroxylapatite. The filler is designed to dissolve over time, but in some cases, it may take months or even years for the body to fully break down.
Precautions should be taken to ensure safe use of temple filler. These include:
- Avoiding physical activities that involve bending, lifting, or heavy exercise for at least 24 hours after treatment
- Avoiding sun exposure and tanning beds during the initial healing period
- Not rubbing or massaging the treated area until it has fully healed
- Avoiding swimming, saunas, and hot tubs for at least 24 hours after treatment
- Following a healthy diet rich in fruits, vegetables, and whole grains to promote overall health and well-being
In the event of an adverse reaction or complication, it is essential to seek immediate medical attention. Early recognition and treatment can help minimize the severity of the problem and prevent long-term damage.
Treatment options for complications associated with temple filler may include:
- Pain management medication or injections to alleviate symptoms
- Surgery to remove any foreign objects, such as a needle or thread, that have become embedded in the skin
- Antibiotics or antiviral medications to treat infections or other inflammatory responses
- Corticosteroid injections to reduce inflammation and swelling
- Cranial CT scans or MRI to evaluate for any damage to the optic nerve or surrounding tissues
In severe cases, surgery may be necessary to repair any damage caused by a cerebral filler spill. This is usually performed under general anesthesia and requires an experienced surgeon who has received specialized training in craniofacial reconstruction.
Avoid using temple fillers as a preventative measure for glaucoma or eye damage. While some people may be at risk for these conditions, there is no conclusive evidence to suggest that temple fillers can prevent them.
However, proper insertion and use of temple fillers, such as silicone oil or gas, can reduce the risk of complications and side effects. It’s essential to follow the manufacturer’s instructions and guidelines for insertion, dosage, and frequency of use.
Prevention is key when it comes to minimizing the risks associated with temple fillers. This includes maintaining a healthy lifestyle, managing any underlying medical conditions, and avoiding other potential eye hazards.
One effective prevention method is to have regular eye exams, ideally every 1-2 years, depending on age, medical history, and risk factors. Early detection and treatment of eye problems can significantly reduce the risk of complications and vision loss.
Treatment options for temple filler-related complications will depend on the individual case and the severity of the issue. In some cases, removal of the temple filler may be necessary to alleviate symptoms and prevent further damage.
Medications such as corticosteroids or cycloplegics may be prescribed to manage inflammation or eye pressure associated with temple fillers. In severe cases, surgery may be required to repair any damage caused by the filler.
A well-known complication of using silicone oil in the eyes is its potential for causing an inflammatory reaction, which can lead to a condition called uveitis.
Uveitis can result in pain and sensitivity to light, blurred vision, and increased eye pressure. If left untreated, this inflammation can cause permanent damage to the retina or other parts of the eye.
The treatment for uveitis typically involves corticosteroid medications, which can help reduce inflammation and alleviate symptoms. In some cases, additional treatments such as cycloplegics or anti-inflammatory medications may also be necessary.
Another potential complication associated with using temple fillers is the risk of developing glaucoma.
Glaucoma is a group of eye conditions that damage the optic nerve, often due to abnormally high pressure in the eyes. In some cases, glaucoma can cause vision loss or even blindness if left untreated.
Treatment for glaucoma typically involves medications such as eye drops or oral medications to reduce eye pressure and prevent further damage.
Proper insertion technique is also crucial when using temple fillers. This includes following the manufacturer’s guidelines for dosing, frequency of use, and any necessary adjustments to avoid complications.
A thorough evaluation by an eye care professional prior to and after insertion can help minimize risks and ensure a safe and effective treatment process.
Additionally, maintaining open communication with your healthcare provider is vital throughout the treatment process. This includes reporting any symptoms or concerns promptly and adhering to prescribed treatments and follow-up appointments.
Proper prevention and treatment options are crucial in minimizing the risk of complications associated with temple filler procedures.
A thorough understanding of the risks involved is necessary to take preventive measures. One of the most significant risks is the formation of granulomas, a type of inflammatory reaction that can cause vision loss or blindness if left untreated.
According to a study published in the Journal of Clinical Ophthalmology, improper insertion techniques are a major contributor to granuloma formation and other complications.
The study highlights the importance of following proper insertion techniques to minimize the risk of complications. This includes using sterile equipment, properly cleaning and disinfecting the area, and inserting the filler material at the correct angle and depth.
Another critical aspect of prevention is patient selection and education. Patients should be thoroughly examined before undergoing a temple filler procedure to ensure they are healthy enough for the surgery.
Potential contraindications include pre-existing eye conditions such as dry eye, glaucoma, or rheumatoid arthritis. Patients with these conditions may require alternative treatment options or special precautions to minimize the risk of complications.
Post-operative care is also essential in preventing complications. Patients should be advised to follow a strict regimen of eye drops and medications to reduce inflammation and prevent infection.
Monitoring for signs of complications, such as redness, swelling, or vision loss, is crucial after a temple filler procedure.
In the event of complications, prompt medical attention is necessary. This may include administration of antibiotics, anti-inflammatory medications, or other treatments to manage symptoms and prevent long-term damage.
Some studies have reported successful treatment outcomes with early intervention and proper management of complications.
A recent study published in the Journal of Ophthalmic Inflammation and Infection found that prompt treatment with intravitreal steroids and antibiotics significantly reduced the risk of vision loss in patients with granuloma formation.
Other treatment options may include surgical removal of the filler material or alternative treatments such as photocoagulation or cryotherapy to manage inflammation.
It is essential for patients to seek medical attention promptly if they experience any signs or symptoms of complications after a temple filler procedure.
A thorough discussion with an eye doctor or plastic surgeon can help determine the best course of treatment and minimize the risk of complications associated with temple fillers.
Ultimately, prevention and treatment options are critical in minimizing the risk of complications associated with temple filler procedures. By following proper insertion techniques, selecting suitable patients, providing comprehensive post-operative care, and seeking prompt medical attention when needed, it is possible to reduce the risk of vision loss or blindness.
Prevention is key when it comes to avoiding complications from temple filler procedures. To minimize the risk of vision problems, it’s essential to choose a qualified and experienced injector who has a track record of successful procedures.
A thorough consultation before the procedure can help identify potential risks. The injector should discuss the potential benefits and risks of the treatment, including the possibility of vision problems, and answer any questions or concerns you may have.
Temple fillers are typically made from hyaluronic acid, a naturally occurring substance in the body that is also found in connective tissue. The filler material should be chosen based on your individual needs and medical history.
During the procedure, the injector will use local anesthesia to numb the area and then inject the filler material into the temple. To minimize the risk of vision problems, it’s essential to choose an experienced injector who uses a sterile technique and follows proper injection protocols.
After the procedure, it’s crucial to follow the injector’s instructions carefully to minimize the risk of complications. This may include avoiding rubbing or touching the treated area, as well as not using makeup or heavy creams for several days.
Regular follow-ups with your injector are also essential to monitor the healing process and address any concerns or issues that may arise. During these visits, your injector can assess the fillers’ placement and adjust them if necessary.
Potential Prevention Measures:
-
Reserve a Dermal Filler Consultation with Dr. Laura Geige Now
- Choose a qualified and experienced injector
- Thoroughly discuss potential risks and benefits with the injector
- Follow proper pre- and post-procedure instructions carefully
- Avoid rubbing or touching the treated area
- Avoid using makeup or heavy creams for several days after the procedure
Treatment Options:
- Hyaluronic acid fillers (e.g., Restylane, Juvederm)
- Silicone fillers (rarely used due to potential risks)
- Permanent fillers (e.g., PMMA, calcium hydroxylapatite)
Treatment Outcomes:
- Pure vision problems (temporary or permanent) can occur if the injector uses poor technique or injects the filler too deeply
- Temporary dry eye, swelling, or redness may occur after the procedure
- Rarely, more serious complications like retinal detachment or stroke can occur (although these are extremely rare)
A well-informed discussion with your injector and a thorough understanding of the potential risks and benefits will help you make an informed decision about temple filler procedures.
No reported cases have been documented, but there is always a possibility that an adverse reaction can occur with any medical procedure.
Prevention measures are crucial to minimize risks associated with temple filler procedures. These measures include choosing a qualified and experienced practitioner who follows proper sterilization techniques, using high-quality equipment, and thoroughly discussing the potential risks and benefits of the treatment with the patient before the procedure.
It is essential for patients to follow pre- and post-operative instructions carefully to ensure optimal results and minimize complications. This includes refraining from certain medications, avoiding heavy lifting or bending, and applying ice packs to reduce swelling in the affected area.
A thorough eye examination prior to the procedure can also help identify any potential eye-related issues that may increase the risk of complications.
Regular follow-ups with an eye care professional are crucial to monitor for any potential complications. This includes scheduling regular check-ups to assess the healing process, address any concerns or questions, and detect any signs of adverse reactions early on.
A comprehensive post-operative care plan should include instructions on how to manage pain, swelling, and bruising, as well as guidance on when to seek medical attention in case of any complications.
Some common potential complications associated with temple filler procedures include eye problems, such as dryness, irritation, or even vision changes. In rare cases, more serious complications like infection or vision loss can occur.
Treatment options for complications will depend on the underlying cause and severity of the issue. For example, antibiotics may be prescribed to treat infections, while anti-inflammatory medications or steroids may be used to manage swelling and inflammation.
In severe cases, surgery may be necessary to repair any damage caused by the procedure. In some cases, vision therapy or rehabilitation may also be required to address any long-term effects of the complication.
Early detection and treatment of complications can significantly improve outcomes and minimize the risk of permanent damage.
A qualified eye care professional should always be consulted if a patient experiences any unusual symptoms, such as blurred vision, double vision, or severe eye pain after the procedure.
The American Academy of Ophthalmology (AAO) recommends that patients follow up with an eye care professional at least 1-2 weeks after the procedure to monitor for any potential complications and address any concerns or questions they may have.
The risk of blindness associated with temple filler injections is a significant concern for those considering this cosmetic treatment. To address this, it’s essential to understand both prevention and treatment options, as well as alternative approaches.
Prevention measures focus on minimizing the risks of complications during and after the procedure:
- Thorough evaluation by an experienced healthcare provider or dermatologist to identify potential contraindications and ensure proper suitability for the treatment;
- Following pre-injection instructions carefully, including any medication to prevent bruising or blood clotting;
- Avoiding blood-thinning medications, such as aspirin or ibuprofen, before the procedure;
- Staying hydrated and rested after the treatment.
Treatment options for blindness caused by temple filler injections are available in various medical specialties:
- Ophthalmology: Specialists in eye care provide emergency evaluations, treatment of complications, and can refer patients to other specialists if needed.
- Plastic Surgery: In cases where the blindness is related to the filler used for cosmetic purposes, plastic surgeons may assist with removal of the filler and rehabilitation of the affected area.
- General Medicine or Family Medicine: Primary care physicians often handle follow-up care after the initial evaluation by specialists.
Alternative options that reduce the risk of blindness include:
- Botox Injections: Used for relaxation and reduction of facial expressions, Botox can be an alternative to temple filler injections for aesthetic purposes.
- Other Aesthetic Treatments: Various treatments like laser skin tightening, chemical peels, or micro-needling offer non-invasive alternatives with fewer risks.
- Surgical Options: Depending on the underlying cause of blindness (e.g., injury to a nerve), surgical procedures may be considered for correction.
It is crucial to note that even with these preventive measures and treatment options, there are still potential complications. If you have concerns about blindness from temple filler injections, consulting an experienced professional as early as possible is vital.
A condition known as chronic conjunctivitis can result from temple filler implant complications.
This chronic inflammatory condition causes scarring to develop on the cornea, leading to vision loss or blindness if left untreated.
Prevention measures may be implemented for individuals who have had temple fillers.
These measures include:
-
Maintaining regular follow-up appointments with an eye doctor
-
Avoiding strenuous activities that could potentially dislodge the implant or cause trauma to the eye
-
Using proper hygiene techniques when handling the temple fillers
Treatment options may include:
Medications:
-
Topical corticosteroids may be used to treat mild cases of chronic conjunctivitis.
-
Oral antihistamines or immunosuppressants may be prescribed for more severe cases.
Surgical intervention:
-
Corneal grafting surgery may be recommended in cases of severe scarring that impairs vision.
-
Extracapsular cataract extraction with an intraocular lens implantation may also be considered for individuals who are blind or severely visually impaired due to temple filler complications.
Alternative options:
-
Scleral lenses may be recommended in certain cases to replace traditional contact lenses, as they do not require the presence of a clear cornea.
-
Corneal implants such as collagen or DMEK (descemet’s membrane endothelial keratoplasty) may also serve as alternative options to avoid using temple fillers altogether.
It is essential to note that the use of these alternative options requires careful consideration and consultation with an eye doctor to determine which option is best suited for a particular individual’s needs.
Frequent follow-up appointments are crucial in monitoring the progression of the condition and adjusting treatment plans accordingly.
A thorough evaluation by an eye care professional is necessary to assess the risks and benefits associated with each option, ensuring that proper treatment can be administered in time.
Regulatory Guidelines and Research Findings
The topic of temple filler-induced blindness raises several regulatory and research-related concerns.
Government regulations play a crucial role in addressing potential health risks associated with certain medical devices, such as temple fillers.
In the context of eye care products, regulatory guidelines are set by agencies like the U.S. Food and Drug Administration (FDA) to ensure that these products meet certain standards for safety and efficacy.
The FDA requires that all eye care products, including those used in surgical procedures such as temple filler implants, undergo rigorous testing before they can be approved for commercial sale.
One of the key regulatory frameworks governing medical devices is the 510(k) clearance process, which evaluates a product’s safety and effectiveness compared to existing devices on the market.
Research findings have also shed light on the potential risks associated with temple filler implants.
A study published in the Journal of Plastic, Reconstructive & Aesthetic Surgery found that one in five patients who underwent temple filler surgery experienced complications, including visual disturbances such as blurred vision and double vision.
Another study published in the American Journal of Ophthalmology discovered that 15% of patients who received temple fillers developed visual loss or other eye problems, including blindness.
The study highlighted the need for stricter regulatory guidelines and more comprehensive research on the long-term effects of temple filler implants.
Furthermore, research has also raised concerns about the potential risks associated with the materials used in temple fillers, such as silicone oil and polyurethane.
A study published in the Journal of Biomedical Materials Research found that these materials can cause chronic inflammation and scarring in the eyes, leading to vision loss or blindness.
Regulatory guidelines should also consider the need for post-marketing surveillance, which involves monitoring patients after a product has been approved for use to identify any potential safety concerns.
This type of surveillance is crucial in identifying rare but serious side effects, such as the ones associated with temple filler implants.
In terms of specific regulations, the FDA requires that all medical device manufacturers report adverse events or other safety concerns related to their products.
The agency also has a mandatory post-market surveillance program for medical devices, which involves monitoring patient outcomes and adverse events over time.
Additionally, some countries have established their own regulatory frameworks for medical devices, such as the European Union’s (EU) Medical Device Regulation (MDR), which sets out stricter guidelines for device safety and effectiveness.
The MDR requires that all medical devices undergo rigorous testing and evaluation before they can be approved for sale in the EU.
Furthermore, research has also highlighted the need for more transparency around the development and approval processes for medical devices, including those used in temple filler implants.
A study published in the Journal of Regulatory Affairs found that a lack of transparency can lead to inadequate oversight and increased risk of adverse events.
This type of transparency is essential in building trust between regulators, manufacturers, and patients, and in ensuring that medical devices are developed and approved with proper safeguards in place.
The use of temple fillers, also known as temple implants or orbital fillers, has gained popularity in recent years for its potential to treat aesthetic concerns such as hollow eyes and facial asymmetry. However, there have been reports of serious complications associated with these procedures, including vision loss and blindness.
Regulatory guidelines surrounding the use of temple fillers are largely based on the FDA’s approval process for medical devices. The FDA requires that any device or medication intended to be used as a filler be proven to be safe and effective through rigorous testing and clinical trials.
In 2010, the FDA approved the first injectable dermal filler specifically designed for use in the orbital area, known as Orbital Fillers by Kybella. However, these fillers were not intended for use in the temple area, and their safety and efficacy in this region have not been thoroughly established.
Research findings on the topic of vision loss and blindness associated with temple fillers are limited. A study published in the Journal of Plastic, Reconstructive & Aesthetic Surgery found that 3 out of 100 patients who underwent orbital fillers experienced significant visual disturbances, including blurred vision, double vision, and light sensitivity.
Another study published in the American Journal of Ophthalmology found that 2 cases of permanent vision loss were associated with the use of temple implants. The study noted that these cases likely resulted from complications such as retinal detachment or optic nerve damage.
The FDA has also issued warnings about the potential risks associated with using fillers in the temple area. In a 2018 warning letter, the agency cautioned against using non-FDA-approved fillers for orbital augmentation, citing concerns about their safety and efficacy.
Furthermore, several professional organizations, including the American Academy of Ophthalmology (AAO) and the American Society of Plastic Surgeons (ASPS), have issued guidelines advising against the use of temple fillers in patients with certain medical conditions, such as glaucoma or retinal detachment.
Additionally, research has shown that the use of fillers in the temple area can lead to a range of complications, including eyelid drooping, facial asymmetry, and scarring. A study published in the Journal of Cutaneous and Aesthetic Surgery found that 75% of patients who underwent temple filler procedures experienced at least one complication.
In light of these findings, regulatory guidelines and research evidence suggest that the use of temple fillers should be approached with caution and carefully considered on a case-by-case basis. Patients considering temple fillers should consult with a qualified healthcare professional to discuss the potential risks and benefits of the procedure.
The FDA has issued guidance on the safe use of temple fillers, emphasizing the importance of proper insertion techniques and follow-up care. The agency’s warning comes amid concerns over the increasing number of reported cases of vision loss and blindness associated with the use of dermal fillers.
According to the FDA, the most common complications associated with temple filler injections include eyelid swelling, bruising, and vision problems. However, in rare cases, more serious complications can occur, including retinal detachment and permanent vision loss.
The FDA has emphasized that improper insertion techniques and inadequate follow-up care are major contributors to these complications. For example, injecting fillers too close to the eye or using excessive amounts of filler material can cause swelling and damage to the delicate tissues around the eye.
Additionally, the FDA notes that patients should be closely monitored by their healthcare providers after receiving temple fillers, with regular check-ups scheduled to ensure that there are no signs of complications. This includes monitoring for redness, swelling, or vision problems.
Research findings have also highlighted the risks associated with temple filler injections. A study published in the Journal of Clinical and Aesthetic Dermatology found that 12% of patients who received dermal fillers experienced some form of vision complication, including blurred vision, double vision, and eye pain.
Another study published in the American Journal of Ophthalmology found that retinal detachment, a rare but serious complication, was associated with the use of facial fillers, including those used for temple fillers. The study suggested that patients who receive these injections should be screened for any signs of retinal detachment before and after treatment.
The FDA’s guidance emphasizes the importance of informed consent and proper training for healthcare providers administering temple fillers. Patients should also be thoroughly educated on the potential risks and benefits associated with these treatments, as well as the need for follow-up care.
Furthermore, regulatory agencies around the world are taking steps to improve the safety and efficacy of dermal fillers, including stricter standards for product testing and approval. For example, the European Medicines Agency has established a centralized database to track adverse reactions associated with dermal fillers.
In the United States, the FDA is also working to improve its oversight of dermal filler manufacturers. The agency has proposed new rules requiring manufacturers to submit data on the safety and efficacy of their products before they can be approved for use in humans.
The use of temple fillers, also known as nose threads or ear piercings, has gained immense popularity over the years, especially among young people. However, there have been reports and warnings raised about the potential risks associated with this practice.
One of the primary concerns is the risk of serious eye complications, including temporary and permanent vision loss. According to a study published in the Journal of Medical Microbiology, there are over 40 known cases of blindness reported worldwide due to temple filler-related injuries.
The most common cause of blindness related to temple fillers is a condition called ” FOREIGN BODY INTRACRISTAL INJURY” (FBIII). This occurs when the thread or piercer enters the eye socket and punctures the delicate skin and tissues, leading to severe inflammation, scarring, and vision loss.
Research has shown that the risk of FBIII is significantly higher in individuals who use high-quality threads with a diameter of 0.2mm or less. A study published in the Journal of Laryngology and Otology found that the likelihood of thread migration into the eye socket increases by 200% when using low-quality threads.
The American Academy of Otolaryngology – Head and Neck Surgery (AAO-HNS) recommends that individuals who plan to undergo ear piercing should choose a reputable piercer and use high-quality threading materials. The AAO-HNS also advises against using threading materials with a diameter larger than 0.3mm.
The Environmental Protection Agency (EPA) has issued guidelines for the safe use of threading materials in medical settings. According to these guidelines, piercing equipment should be sterilized before each use, and threads should be made from hypoallergenic materials that are resistant to corrosion and microbial growth.
A study published in the Journal of Clinical and Aesthetic Dermatology found that 75% of ear piercers surveyed reported using unsterilized equipment on at least one occasion. This highlights the need for strict adherence to sterilization protocols to prevent the transmission of infections and other complications.
The National Institute for Occupational Safety and Health (NIOSH) has also issued guidelines for the safe handling and use of threading materials in medical settings. According to NIOSH, proper ventilation, personal protective equipment, and regular training are essential for minimizing exposure to biohazards and other occupational risks.
Despite these warnings and guidelines, many people continue to ignore the risks associated with temple fillers. It is essential that individuals take a responsible approach to ear piercing, prioritizing their safety and well-being above any aesthetic or social concerns.
In conclusion, while temple fillers may seem like a harmless practice, they carry significant health risks that should not be underestimated. By understanding the potential complications and taking steps to mitigate them, we can reduce the risk of blindness and other serious eye injuries.
The use of _temporal filler_ (TF) implants has been a subject of concern for otorhinolaryngologists and researchers in recent years, particularly in relation to potential complications that can lead to serious outcomes such as vision loss or even blindness.
According to the Environmental Protection Agency (EPA), regulatory guidelines suggest exercising caution when using TF implants in individuals with pre-existing medical conditions, including those with a history of _eye problems_ or allergies. This warning is based on research findings that have identified a possible link between the use of TF implants and an increased risk of adverse reactions in susceptible individuals.
One key study published in the JAMA Ophthalmology journal investigated the long-term outcomes of patients who underwent TF implantation for hearing restoration. The researchers found that a subset of patients with pre-existing _ocular surface disorders_ (such as dry eye or conjunctivitis) were at higher risk of developing _sensory deprivation syndrome_ (SDS), a condition characterized by visual disturbances, including blurred vision and loss of peripheral vision.
The study’s findings suggest that individuals with a history of _eye problems_, particularly those involving the cornea or ocular surface, may be more susceptible to adverse reactions from TF implants. This is because these pre-existing conditions can compromise the body’s ability to regulate the surrounding tissues and fluids, increasing the risk of complications when introducing a foreign object like a TF implant.
Another study published in the Journal of Laryngology and Otology examined the relationship between TF implantation and the development of _allergic reactions_. The researchers discovered that patients with a history of allergies, particularly those involving the eyes or ears, were more likely to experience adverse reactions to TF implants, including increased sensitivity to light, itching, or redness in the affected areas.
These studies and others have led regulatory agencies like the EPA to recommend avoiding the use of TF implants in certain individuals, such as those with a history of _eye problems_ or allergies. It is essential for patients considering TF implantation to disclose their pre-existing medical conditions and any relevant allergies to their surgeon, allowing them to make an informed decision about their treatment options.
In addition to the research findings mentioned above, there have been several case reports and studies documenting instances of vision loss and blindness following TF implantation. These cases often involve patients with pre-existing eye problems or those who have experienced an adverse reaction to the implant itself. In some cases, the vision loss is irreversible, highlighting the importance of careful patient selection and monitoring when using TF implants.
The use of temple fillers, also known as tympanostomy tubes, has been a common practice for decades to treat middle ear infections and other conditions affecting hearing. However, there have been concerns raised about potential side effects, including vision problems.
Research studies have investigated the relationship between temple filler insertion and blindness or significant vision loss in children and adults. While some studies have reported associations between tympanostomy tubes and visual impairment, others have found no link or concluded that any observed effects are likely due to other factors.
- A 2013 systematic review of 23 studies on tympanostomy tubes published in the Journal of Pediatric Otolaryngology-Head and Neck Surgery found no significant association between tube insertion and blindness or vision loss in children.
- A 2020 study published in the American Journal of Ophthalmology: Ophthalmic Pathology & Imaging found that among a cohort of over 4,500 children who had undergone tympanostomy tube insertion, there were no cases of blindness or significant visual impairment.
- However, some observational studies have reported associations between tympanostomy tubes and vision problems. For example, a 2017 study published in the journal BMJ Open found that among over 1,000 children who had undergone tube insertion, there was an increased risk of developing amblyopia (lazy eye) compared to those without tubes.
Research findings suggest that any association between temple fillers and blindness or vision loss is likely to be rare. However, the long-term effects of tympanostomy tube insertion on visual development in children are not yet fully understood, and more research is needed to address this question.
A 2019 study published in the Journal of Children’s Health Care found that among a cohort of over 1,000 children who had undergone tube insertion at age 3 years, there were no significant differences in visual acuity or visual fields compared to children without tubes by age 6 years. However, the study also noted that follow-up studies would be needed to determine whether any potential effects on vision development become apparent later in childhood.
In terms of regulatory guidelines, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) recommends routine tympanostomy tube insertion for children with recurrent acute otitis media or other conditions that require tube placement. The AAO-HNS also emphasizes the importance of monitoring for any potential side effects, including vision problems.
The FDA has not issued any specific warnings about the risk of blindness or significant vision loss associated with tympanostomy tubes. However, the agency does recommend that parents and healthcare providers closely monitor children who have undergone tube insertion for any signs of visual impairment, such as blurred vision, double vision, or eye pain.
Overall, while some research studies have reported associations between temple fillers and vision problems, the evidence is not yet conclusive, and more research is needed to fully understand the long-term effects of tympanostomy tube insertion on visual development in children.
The use of *_ temple fillers_* has become increasingly popular over the years, with many individuals opting for these cosmetic procedures to enhance their appearance. However, there have been several reports and studies conducted on the potential risks associated with temple fillers, including blindness.
One of the main causes of vision loss or blindness related to temple fillers is *_infection_*. This occurs when bacteria or other microorganisms enter the body through improper injection techniques or contaminated equipment. In some cases, the infection can spread to the surrounding tissue and cause damage to the *_optic nerve_*.
Another possible cause of vision loss is *_vascular occlusion_*, which occurs when a blood vessel becomes blocked due to the filler material. This can lead to ischemia, or reduced blood flow, to the optic nerve, resulting in blindness. In some cases, the blockage may be temporary and resolve on its own, but in other instances, it can cause permanent damage.
Research has also shown that *_ Foreign Body Reactions_* can occur when the body reacts to the foreign material used in temple fillers. This reaction can lead to inflammation, scarring, and damage to surrounding tissue, including the *_cornea_*.
A study published in the *_Journal of Clinical Aesthetic Surgery_* found that between 2007 and 2015, there were 247 reported cases of blindness or vision loss associated with temple fillers. The study noted that the majority of these cases occurred within three months after the procedure and were caused by *_infection_*, *_vascular occlusion_*, or *_foreign body reactions_*.
A review article published in the *_American Journal of Ophthalmology_* highlighted the potential risks associated with temple fillers, including blindness. The authors noted that while the overall incidence of vision loss is low, it can be significant in certain cases, particularly those involving *_infection_* or *_vascular occlusion_*. The authors also emphasized the importance of proper training and technique among practitioners performing these procedures.
Another study published in the *_British Journal of Ophthalmology_* investigated the long-term effects of temple fillers on vision. The researchers found that while many patients experienced no significant changes in their vision, a small percentage developed *_persistent vision problems_*, including blurred vision and sensitivity to light. In some cases, these issues persisted for several years after the procedure.
The exact incidence of blindness caused by temple fillers is difficult to determine due to the lack of comprehensive reporting and tracking. However, it is clear that this risk exists, particularly if proper precautions are not taken during the procedure or if substandard equipment is used.
To minimize the risk of vision loss associated with temple fillers, patients should carefully research their practitioner’s qualifications and follow pre- and post-operative instructions closely. Additionally, patients should be aware of the potential risks and complications associated with these procedures and report any concerns to their practitioner promptly.
The use of temple fillers, also known as implants or prosthesis, has become increasingly popular over the years to restore vision and improve the appearance of individuals with cataracts or other eye conditions. However, research studies have revealed various potential risks and complications associated with these devices.
Granuloma formation is one of the reported side effects of temple fillers, where a group of immune cells forms a foreign body reaction to the implant, leading to inflammation and scarring (1). This condition can cause pain, redness, and swelling in the affected eye, and if left untreated, may lead to chronic discomfort and vision problems.
Optic nerve damage is another potential risk of temple fillers. Studies have shown that the pressure exerted by the implant on surrounding tissues can cause damage to the optic nerve, leading to long-term vision loss and other complications (2). This type of damage can be irreversible, resulting in permanent vision impairment.
Research studies have also identified a potential link between temple fillers and endophthalmitis, a rare but serious infection that affects the interior of the eye. This condition can cause severe pain, swelling, and vision loss if left untreated (3).
A study published in the Journal of Cataract and Refractive Surgery found that 12% of patients who underwent temple filler implantation experienced complications related to the device, including granuloma formation and optic nerve damage (4). The study concluded that while the benefits of temple fillers in restoring vision outweigh the risks, it is essential for patients to be aware of these potential complications.
Another research study published in the Ophthalmic Surgery, Lasers, and Imaging: Retina found that patients who received temple fillers were at increased risk of developing cataracts in the implanted eye compared to those who did not receive the implant (5). This increased risk highlights the importance of careful patient selection and surgical technique when using temple fillers.
In summary, research studies have identified various causes and effects associated with temple fillers, including granuloma formation, optic nerve damage, long-term vision loss, endophthalmitis, and cataract development. While these complications are relatively rare, it is essential for patients to be aware of the potential risks and benefits of temple filler implantation to make informed decisions about their eye care.
The use of temple fillers, also known as injectable materials used to fill hair loss gaps, has become increasingly popular in recent years. However, concerns have been raised regarding the potential long-term consequences of these treatments.
Regulatory guidelines have evolved to address these concerns. In 2019, the US FDA issued a warning about the risks of permanent vision loss associated with injectable fillers used for hair transplantation and other medical purposes. The agency advised healthcare professionals to exercise caution when using these products and to follow proper guidelines.
Research findings suggest that temple fillers can cause a range of complications, including:
- Vision loss or blindness due to the insertion of materials into the eye or surrounding areas
- Infection and inflammation at the injection site
- Foreign body reactions, such as granulomas or nodules, at the injection site
- Scarring and disfigurement
- Permanent damage to the surrounding tissue or nerves
A study published in the Journal of Dermatological Surgery and Oncology found that among 157 patients who received temple fillers, 12 (7.6%) experienced permanent vision loss or blindness. The study suggested that proper training, experience, and adherence to guidelines are crucial in minimizing the risk of complications.
The American Academy of Ophthalmology has also issued guidelines for the use of injectable materials in ophthalmic procedures, including temple fillers. The organization emphasizes the importance of:
- Using only FDA-approved products
- Following proper injection techniques and protocols
- Conducting thorough patient evaluations and informed consent
- Monitoring patients for potential complications
In addition, the American Society of Plastic Surgeons recommends that patients undergoing temple filler procedures be counseled on the potential risks and benefits of treatment. The society also emphasizes the importance of using only qualified healthcare professionals who have received proper training in the use of these products.
The long-term consequences of temple fillers can vary widely depending on individual factors, such as the type of material used, the skill of the practitioner, and the overall health of the patient. However, it is essential to be aware of the potential risks and take steps to minimize them. Patients who are considering temple filler procedures should carefully weigh the benefits against the potential drawbacks and discuss their concerns with a qualified healthcare professional.
Temple fillers, also known as facial injectables, have been a popular cosmetic treatment for years, promising to reduce the appearance of fine lines and wrinkles on the face. However, like any medical procedure, there are potential risks associated with temple filler injections.
One of the longterm consequences of using temple fillers is chronic inflammation. This can occur when the body’s immune system reacts to the foreign substance in the filler, causing swelling, redness, and pain in the treated area.
Another potential risk is scarring. While rare, it is possible for temple fillers to cause permanent scarring, especially if the skin is not suitable or if the filler material is not injected properly.
Permanent vision loss is a potentially serious and longterm consequence of using temple fillers. This can occur when the filler migrates from its original injection site and enters the orbit or eyelid, causing damage to surrounding tissue and vision problems.
Here are some research findings that highlight the risks associated with temple fillers:
- A study published in the Journal of Clinical and Aesthetic Dermatology found that chronic inflammation was a common complication of facial filler injections, affecting up to 30% of patients.
- A review of 15 cases published in the Aesthetic Surgery Journal reported scarring as a potential complication of temple fillers, with a reported incidence of 3.5%.
- A case series published in the journal Ophthalmic Plastic and Reconstructive Surgery documented three cases of permanent vision loss associated with facial filler injections, highlighting the need for caution when injecting near the eye area.
It’s worth noting that these risks can be minimized by choosing a qualified and experienced healthcare professional to administer temple fillers. Additionally, proper aftercare and monitoring can help identify potential complications early on.
While temple fillers can provide effective results for many people, it’s essential to be aware of the potential longterm consequences and take steps to mitigate them. Patients should carefully weigh the benefits against the risks before undergoing treatment and follow post-treatment instructions to minimize the risk of complications.
It’s also worth noting that newer technologies and materials are being developed to reduce the risk of complications associated with temple fillers. For example, some injectables are designed to be more biocompatible or to reduce inflammation, but more research is needed to fully understand their safety and efficacy.
Read more about Tattoo Culture Magazine here. Read more about The Fine Nanny here. Read more about The New Cinema Magazine here. Read more about Arielle Likes to Cook here.
- Traptox Aka Trapezius Botox Treatment Near Sanderstead, Surrey - January 8, 2025
- Skin Pen Microneedling Near Chiddingfold, Surrey - January 6, 2025
- Skin Pen Microneedling Near Reigate, Surrey - January 4, 2025